Eli Lilly Licenses $200m ADC Tech from Immunogen

In an SEC filing published earlier this week, Immunogen has said it has granted Lilly rights to use its maytansinoid-based Targeted Antibody Payload (TAP) technology to develop and commercialize products directed to a specific antigen target.
The license comes after the two firms inked a three-year Multi-Target Agreement in December 2011 that saw a $20m (€15.1m) upfront payment from Lilly to have the right-to-test Immunogen’s TAP technology with its own antibodies.
“Under the terms of the [original] agreement, Lilly took an exclusive development and commercialization license to a single target,” Immunogen said in its annual report filing, released yesterday.
“For the first development and commercialization license taken, which occurred in August 2013, we are entitled to receive up to a total of $200.5 million in milestone payments, plus tiered royalties in the mid-single to low-double digits on the commercial sales of any resulting products.”
Lilly will be responsible for the manufacturing, product development and marketing of any products resulting from this collaboration, Immunogen said.
TAP Tech
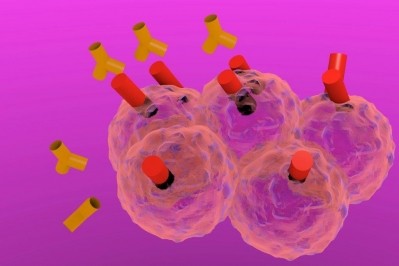
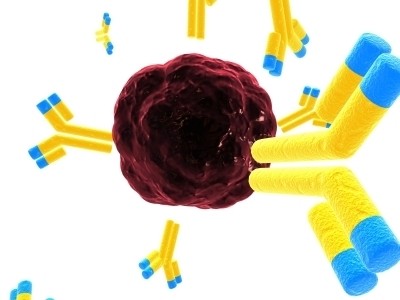
Immunogen’s TAP technology involves attaching potent small-molecule cytotoxics to antibodies for targeted delivery to cancer cells. Furthermore, engineered linkers keep the compound attached to the antibody controlling release in in order to reach the target cancer cells in a patient's bloodstream.
According to the firm, its technology has led to more clinical data being published on more anticancer compounds than with any other ADC technology.
Lilly is not the only Big Biopharma to be collaborating with Immunogen. Sanofi, Novartis, Bayer and Amgen are all currently on its books, as is Genentech who saw regulatory success earlier this year with approval of its metastatic breast cancer drug Kadcyla – developed using Immunogen’s platform - from the US Food and Drug Administration (FDA).
This was the first ADC approved for a solid tumor indication by the FDA and only the second commercially available ADC in the US after Seattle Genetic’s Adcetris in 2011.